28+ Why Metals Are Good Conductors
All metals do not have equal. Web Metals have low resistance and high electrical conductivity.

Metals Good Conductors Of Heat Mov Youtube
Metals are a conductor of electricity because the electrons are free to move in a network of metal atom.

. Web Metals are good conductors of electricity because they have relatively more free electrons to move. Web The reason why metals are good conductors has to do with the nature of their electrons. The free electrons can move due to the potential difference across the metals.
When a potential is applied across any 2 ends to a metal the free. Web Metals are good conductors of electricity because of their atomic structure that allows electric charges to pass through freely. Metallic bonding causes metals to conduct electricity.
Web Why metal is a good conductor. Bad conductors comprise substances that do not. Silver is one of the best metals for conducting heat because it works as a powerful reflector.
Web Metals are good conductors of electricity because the atoms in the metal create a metallic bond due to which the electrons can move in a free state. Web Metals are good conductors of electricity. The free electrons allow a.
The electrons can move to carry the charge electricity. Metal works better as a conductor than wood or plastic because of the presence of conduction. Web Good and bad conductors of electricity.
Web Metals are good conductors of electricity since they have a sea of delocalised electrons they are free to move. Why is metal a better conductor than wood or plastic. In a metallic bond atoms of the metal are surrounded by a constantly moving sea of.
Web Metals are good conductors of current and heat because of its metallic bonding which provides large number of free electrons in it. Metal is one of the best conductors as atoms in the metal make a matrix this is how outer electrons move. Web Metals That Conduct Heat the Best.
The outer or valence electrons in metals are shared by all the atoms. Good conductors include elements that allow electricity to move freely through them. Metals are good conductors because they consist of lattice of atoms with free electrons.
The atomic structure of metals is.
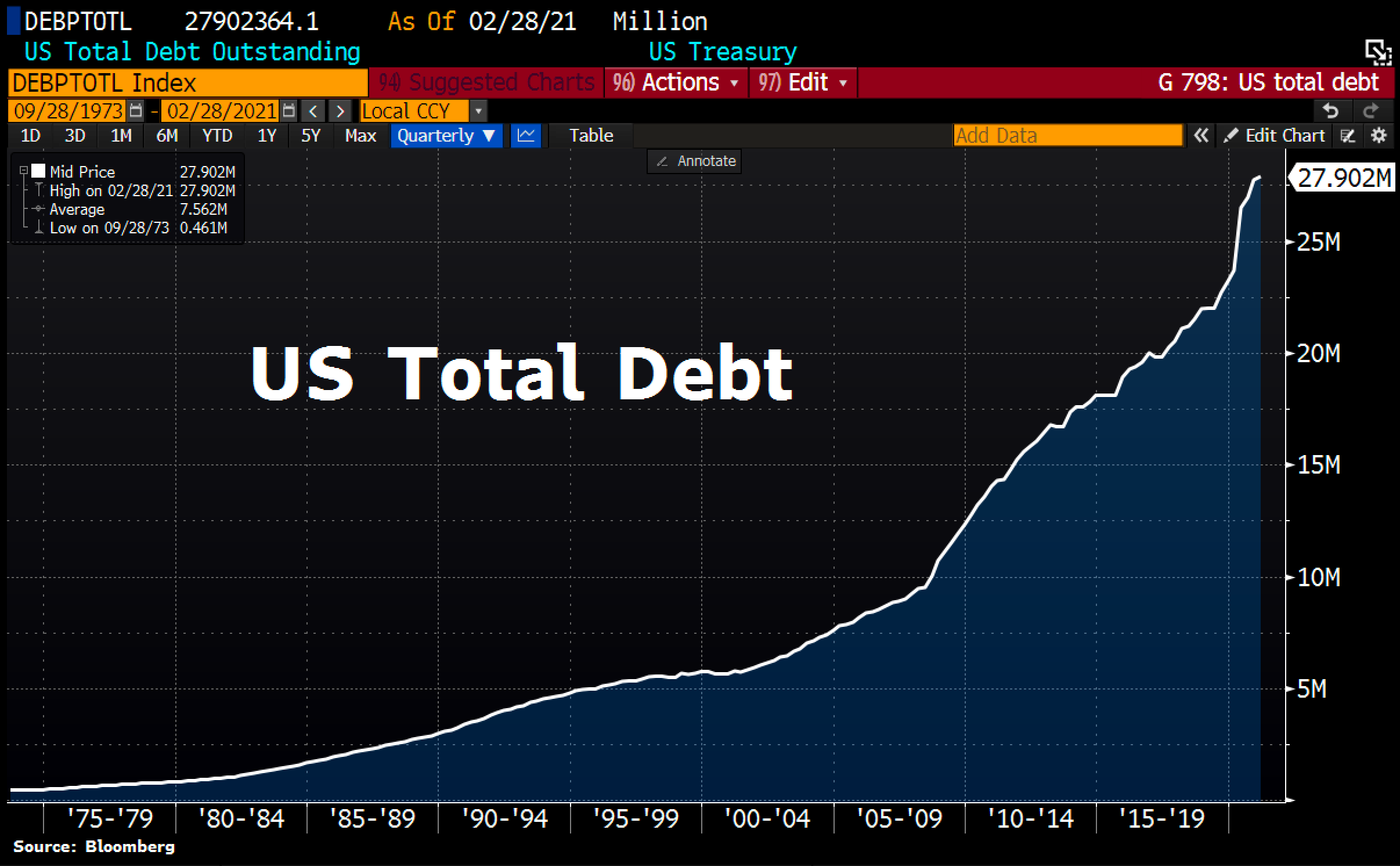
The Metrics Of Silver Squeeze How Are We Doing Renaissancemen Org
Corona Rings Grading Rings Corona Rings Manufacturers

Transition Metal Catalysis Controlled By Hydrogen Bonding In The Second Coordination Sphere Chemical Reviews

Why Are Metals Good Conductors Of Electricity A Closer Look

Properties Of Metals Metals Are Good Conductors Of Heat And Electricity Metals Are Shiny Metals Are Ductile Can Be Stretched Into Thin Wires Metals Ppt Download
What Makes A Metal A Good Conductor Why Is Gold A Good Conductor When Compared To Iron Quora
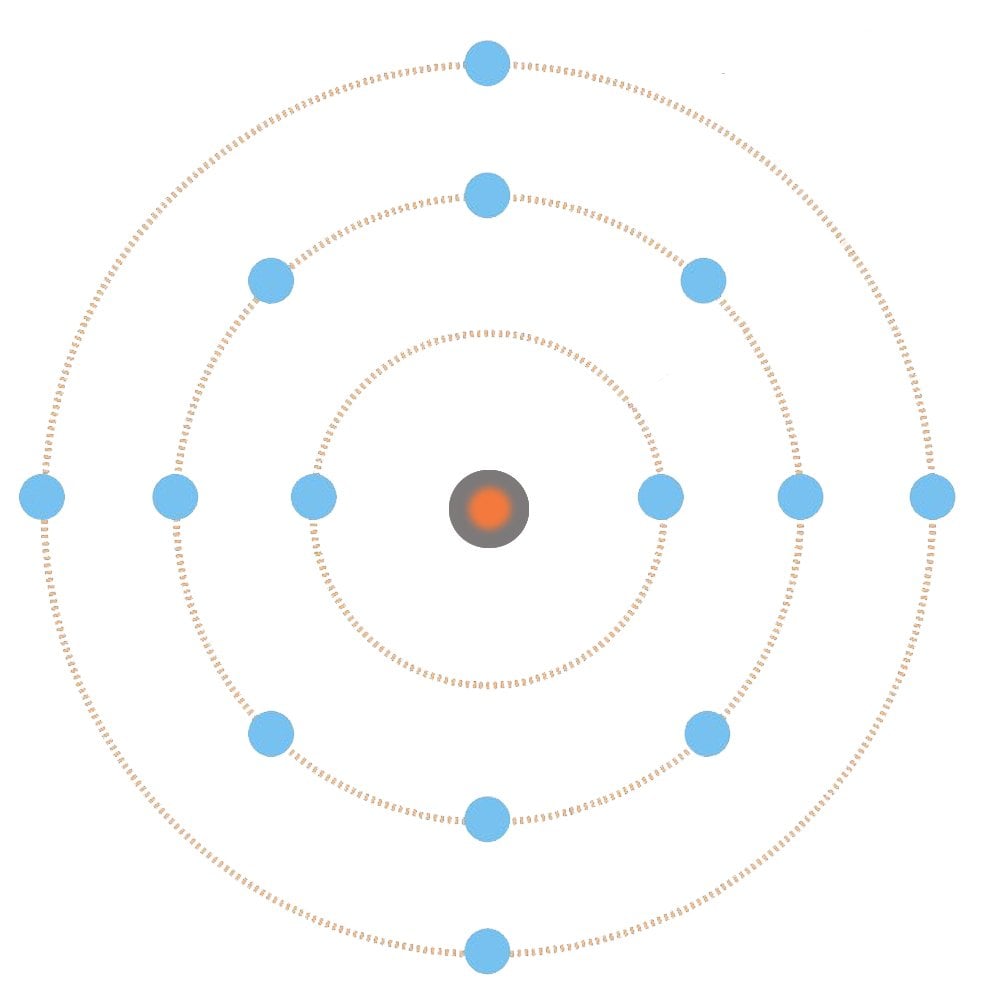
Why Are Metals Good Conductors Of Electricity And Heat

Class 10 Metals And Non Metals

Write An Activity To Prove Metals Are Good Conductors Brainly In

The Reasons Why Metals Are Such Good Conductors Engineer Fix

Transition Metal Catalysis Controlled By Hydrogen Bonding In The Second Coordination Sphere Chemical Reviews

Why Are Metals Good Conductors Of Electricity And Heat

Wis Hiliter 9 24 14 By Edward Nadolski Issuu
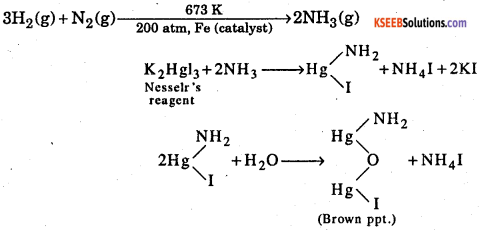
1st Puc Page 57 Kseeb Solutions

Molecular Cavity For Catalysis And Formation Of Metal Nanoparticles For Use In Catalysis Chemical Reviews

Voliitspart Iii Pdf Wire Electrical Conductor
Prasanna Page 301 Kseeb Solutions